Nitric Acid Formula is given by HNO3 where it's one molecule contains 3 oxygen atoms, 1 nitrogen atom, and 1 hydrogen atom.
Nitric Acid Formula is HNO3 where the nitrogen atom is bonded to a hydroxy group and by equivalent bonds to the remaining two oxygen atoms. Nitric acid, an inorganic compound, is a highly corrosive mineral acid.
What is Nitric Acid?
Nitric acid is the most important and useful oxoacid of nitrogen with the chemical formula HNO3. Also known as the spirit of nitre and aqua fortis, it is colourless in its pure form but turns into a yellow cast as it gets older. This colour change is due to the decomposition of nitric acid to oxides of nitrogen and water.
Nitric acid is highly corrosive, toxic, and a very strong oxidising agent. It causes severe skin burns. It has a molar mass is 63.01g/mol and its PH value is approximately 3.01. HNO3 reacts with metals, oxides, and hydroxides to form nitrate salts. It can also be manufactured by the catalytic oxidation of ammonia. Nitric acid (HNO3) is a common reagent used in laboratories and is also an important chemical used in the manufacture of explosives and fertilizers.
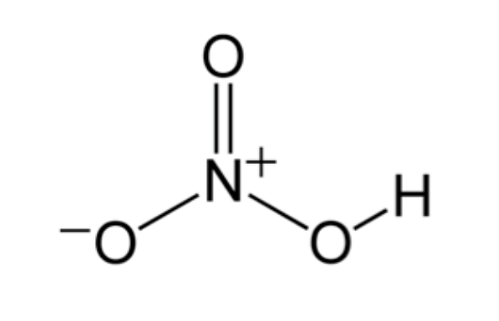
Structure of HNO3 Molecules
The nitric acid formula is HNO3. One molecule of nitric acid contains 3 oxygen atoms, 1 nitrogen atom, and 1 hydrogen atom. The shape of the HNO3 molecule is planar, with the nitrogen atom attached to three oxygen atoms, out of which one holds the proton. The other two N-O (nitrogen-oxygen) bonds are equivalent and show resonance with a double bond character.
In nitric acid (HNO3) molecules, one of the oxygen atoms is doubly bonded to the nitrogen atom at the centre. The other oxygen atom is singly bonded to both the central nitrogen atom and a hydrogen atom. The last oxygen atom in the nitric acid molecule has a negative charge of -1 and is singly bonded to the central nitrogen atom. Since the nitrogen atom at the centre of the HNO3 molecule is participating in four covalent bonds with 3 oxygen atoms, it has a charge of +1.
Hence, the net charge on the HNO3 molecule is 0 as the positive charge on the nitrogen atom and the negative charge on the oxygen atom cancel each other out. It must be noted that the charges in these molecules can be delocalized due to the resonance effect. The structure of nitric acid molecules is shown below.
Laboratory Preparation of Nitric Acid
Nitric acid is generally prepared in the laboratory by heating potassium nitrate or sodium nitrate with concentrated sulphuric acid. The heating process is done in a glass retort, and the vapours of nitric acid are condensed in a receiver which is cooled by water.
Principle
A principle followed in the laboratory preparation of nitric acid is that a more volatile acid can be displaced from its salt by a less volatile acid.
Illustration
Nitric acid is a more volatile acid compared to sulphuric acid and is displaced by sulphuric acid from metal nitrates.
Reactants
50gm of potassium nitrate (KNO3) + 25ml of concentrated sulphuric acid (H2SO4) are taken in a round-bottomed flask. The heat of about 200°C is applied ensuring that the temperature does not cross 200°C.
The Reaction
KNO3 + Concentrated H2SO4 + Heat → KHSO4 + HNO3
(Salt of more volatile acid + less volatile acid → displaces more volatile acid)
Apparatus Setup
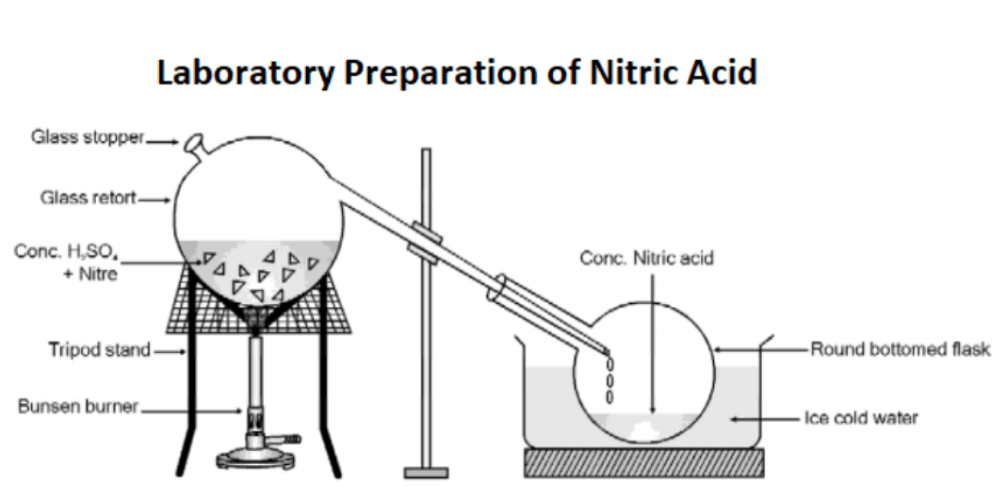
Method of Collection
The vapours of nitric acid are cooled and condensed for collection as demonstrated in the above diagram.
Properties of Nitric Acid - HNO3
Nitric acid is a colourless, fuming, and highly corrosive liquid (with a freezing point of -42 °C [-44 °F] and boiling point of 83 °C [181 °F]). Find below the physical and chemical properties of nitric acid in detail:
Physical Properties of Nitric Acid - HNO3
Nitric acid appears as a pale yellow to reddish brown liquid generating red-brown fumes and has an acrid, pungent and suffocating odour. Tabulated below are some of the physical properties of HNO3:
| HNO3 | Nitric Acid |
| PH Value of Nitric Acid | 3.01 |
| Molecular Weight/ Molar Mass | 63.01 g/mol |
| Density | 1.51 g/cm³ |
| Boiling Point | 83 °C |
| Melting Point | -42 °C |
Chemical Properties of Nitric Acid - HNO3
Nitric acid is strong and monoprotic acid widely used in digestion reagents and the most widely used as a primary oxidant for the decomposition of organic matter. Find below some of the chemical properties of HNO3:
- Nitric acid is a very strong acid that turns blue litmus red.
- It decomposes on standing to form brown nitrogen dioxide. This is why it becomes brownish (yellowish) over time though fresh nitric acid is colourless.
4HNO3 → 4NO2 + O2 + 2H2O
- It frequently forms solid hydrates like monohydrate HNO3⋅H2O and the trihydrate HNO3⋅3H2O.
- It is a powerful oxidizing agent and reacts violently with several non-metallic compounds. It also reacts with metals and metals dissolve in it to produce metal oxides.
- Nitric acid liberates hydrogen gas with metals above hydrogen element 0 in the metal activity series.
Mg + 2HNO3 → Mg(NO3)2 + H2
Mn + 2HNO3 → Mn(NO3)2 + H2
Uses of Nitric Acid
Nitric acid is used for the production of ammonium nitrate which is a major component of fertilizers. It is also used for the production of explosives such as nitroglycerin and trinitrotoluene (TNT) and for oxidizing metals. Listed below are a few other uses of nitric acid:
- Used to produce ammonium nitrates used in the manufacturing of plastic, dye, and fertilizers
- Used in making explosives such as TNT
- Used in liquid-fueled rockets as an oxidizer
- Used in the removal of the wart in its purest form
- Used in electrochemistry as a chemical doping agent