CBSE 12th Board Exams Commence Soon: Check CBSE 12th Physics Chapter 12 Important Questions
The Central Board of Secondary Education officials will be conducting the class 12 Board exams from Feb 15 till Apr 5, 2023. The physics exam is scheduled to be held on Mar 6, 2023.
CBSE 12th Physics Chapter 12 Important Questions: The Central Board of Secondary Education officials will conduct the class 12 Board exams from Feb 15 till Apr 5, 2023. The candidates preparing with CBSE 12th Physics Chapter 12 important questions must note that the Physics exam is scheduled to be held on Mar 6, 2023.
Subsequently, the candidates must focus on CBSE 12th Physics important questions from a preparation point of view. It is highly advised for all the applicants to prepare for CBSE 12th Physics chapter about Atoms. Further, for CBSE 12th Physics Revision, a thorough study of the CBSE 12th physics atoms would ensure a better score for the students.
Also Read: CBSE 12th Physics Chapter 11 Important Questions
CBSE 12th Physics Chapter 12 Important Questions
Candidates must refer to the following to prepare for the CBSE 12th Physics Chapter 12 important questions.
- Question 1. What is the value for ionisation energy for a hydrogen atom? Define.(All India 2010)
Answer: The energy required to knock out an electron from an atom is called ionisation energy of the atom. Moreover, for hydrogen atom it is 13.6 eV. - Question 2. State the expression for Bohr’s radius in hydrogen atom. (Delhi 2010)
Answer: Bohr’s radius in hydrogen atom,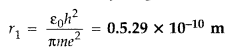
- Question 3: While corresponding to first excited state and ground state in a hydrogen atom, what is the ratio of radii of the orbits? (Delhi 2010)
- Answer:

- Question 4: What is the radius of orbit in the second excited state, if the radius of innermost electron orbit of a hydrogen atom is 5.3 × 10-11 m. (Delhi 2011)
Answer: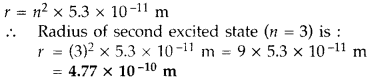
- Question 5: In the given situations, find the ratio of energies of photons produced due to transition of an electron of hydrogen atom from; (i) second permitted energy level to the first level, and (ii) the highest permitted energy level to the first permitted level. (All India 2010)
Answer: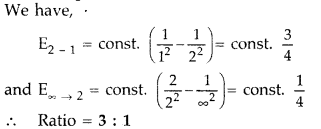
- Question 6: What are the kinetic and potential energies of electron in this state if the ground state energy of hydrogen atom is -13.6 eV. (All India)
Answer: Kinetic energy, Ke = + T.E. = 13.6 eV, Potential energy, Pe = 2 T.E. = 2 (-13.6) = – 27.2 eV - Question 7. Unable to explain the atomic structure, why is the classical (Rutherford) model for an atom—of electron orbitting around the nucleus? (All India 2012)
Answer: As the revolving electron loses energy continuously, it must spiral inwards and eventually fall into the nucleus. So it was not able to explain the atomic structure.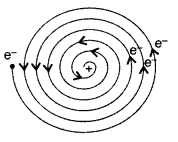
- Question 8: Explain how the Ha line of the Balmer series in the emission spectrum of hydrogen atom is obtained? (Comptt. Delhi 2012)
Answer: Balmer series is obtained when an electron jumps to the second orbit (n1 = 2) from any orbit n2 = n > 2 . - Question 9: In the third excited state, what is the maximum number of spectral lines emitted by a hydrogen atom? (Comptt. All India 2012)
Answer: For third excited state, n2 = 4, and n1 = 3, 2, 1 Hence there are 3 spectral lines. -
Question 10: Derive the expression for the total energy of the electron in hydrogen atom using the Rutherford model of the atom. Explain what the significance of total negative energy possessed by the electron is? (All India 2012)
Answer: Expression for total energy of electron in H-atom using Rutherford model : As per Rutherford model of atom, centripetal force (Fc) required to keep electron revolving in orbit is provided by the electrostatic force (Fe) of attraction between the revolving electron and nucleus.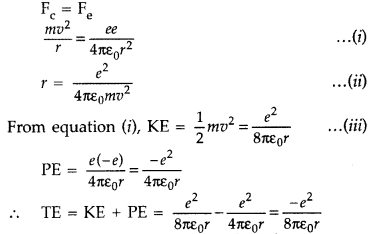
The negative sign indicates that the revolving electron is bound to the positive nucleus.




