The mass of electron is the mass of a stationary electron described by the equation me = 9.10938356 × 10-31 kilograms where me is the rest mass of electron.
Table of Contents
The mass of electron is 1/1836 of the proton mass, denoted by me = 9.10938356 × 10-31 kilograms. It is the mass of a stationary electron and is also known as the invariant mass of electron. The mass of an electron is a fundamental constant of physics expressed in various units like amu (atomic mass units), eV (electro volts), etc.
Value of Mass of Electron
As per the special theory of relativity, the mass of any object varies according to the frame of reference. Therefore, the mass of electron can also be considered as rest mass which has an energy equivalent of approximately 8.187 × 10-14 Joules. Electrons have a coefficient of -1 and weigh 0 ounces (atomic mass unit) around the nucleus.
Charge, mass, spin, and other properties are passed down to an electron. The electron charge, mass, and quantum mechanical properties known as electron spin are all represented in terms of their respective units by the values of electrons.
Charge of Electron
The electric charge by an Electron is the unit elementary negative charge.
The value is: e– = 1.60217662 × 10-19 coulombs
The charge of an electron is responsible for the electric charge.
Mass of Electron
The mass of an electron is 1/1836 of proton mass.
The value of electron mass is: me = 9.10938356 × 10-31 kilograms
The values of charge and mass of electrons are commonly used in solving problems in physics.
Electron Spin
Spin or angular momentum are intrinsic quantum mechanical properties of subatomic particles.
The spin of an electron is half-integral values: s = ½
Rest Mass of Electron
The mass of a stationary electron can be calculated through spectroscopic measurement and the Rydberg Constant. The formula for the mass of electron is shown below:
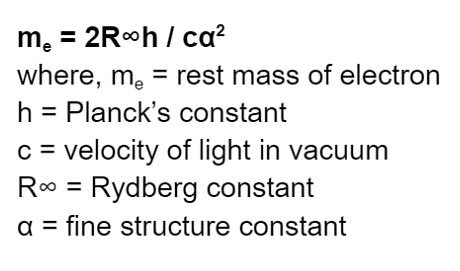
The values of the mass of electrons in different units are shown in the table below:
| Units | Mass of Electron |
| kg | 9.10938356 (11) × 10-31 |
| grams | 9.10938356 (11) × 10-28 |
| amu | 5.48579909070 (16) × 10-4 |
| ev | 0.5109989461 (31) |
Determination of Charges for Mass Ratio of Electrons
Electrons were discovered back in the 19th century by J.J Thomson when he proposed Thomson’s Atomic Model. An experiment was conducted to determine the charge and mass of electrons.
The charge-to-mass ratio of the electrons was determined by conducting accurate measurements on the number of deflections observed by the electrons in the electric field and magnetic field. The charge-to-mass ratio of electrons is given by,
e/m = 1.758820 × 1011 C/kg
where m = mass of electron = 9.10938356 × 10-31 kg
e = charge of the electron = 1.602 × 10-19 coulombs
Experimental Set-Up to Determine Charges for Mass Ratio of Electron
J.J. Thomson conducted a discharge tube experiment in which he observed that the particles in the cathode showed a deviation from their path. The extent of this deviation depends upon the different parameters mentioned below:
- The particles with a higher magnitude of the charge experienced greater interaction with the electric and magnetic field and thus showed greater deflection.
- The particles with a lower mass also experienced greater deflection. Therefore, deflection is inversely proportional to the mass of electron.
- The deflection shown by the particles is directly proportional to the strength of the electric and magnetic fields.























POST YOUR COMMENT