The relation between molarity and molality is expressed by m=1000M / 1000ρ−MM1 where ρ is the density of solution (mg/mL) and M1 is the molecular weight of the solute.
Table of Contents
The relation between molarity and molality is given by m=1000M / 1000ρ−MM1 where ρ is the density of solution (mg/mL) and M1 is the molecular weight of the solute. Both molarity and molality are terms used to quantitatively express the strength of the solution (concentrated or diluted). Molarity is the concentration of a solution expressed in terms of moles per unit volume whereas molality is the concentration expressed in terms of moles per unit mass.
Relation Between Molarity and Molality
The relation between molarity and molality is that both play a crucial role in the determination of the concentration of a solution. The derivation of the relation between molarity and molality is given below.
Let us suppose the mass of the given solute is W g (W / 1000kg)
Let the mass of the given solute be ‘W’
Let the volume of the solution be ‘V’
Let the molality be ‘m’
Let the molar mass of solute be ‘M1’
Let the Molarity be ‘M’
Let the weight of the solvent be ‘W1’
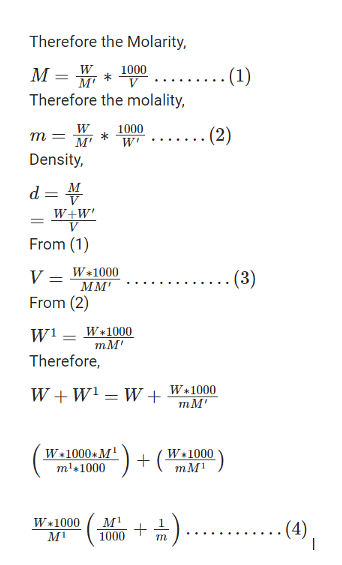
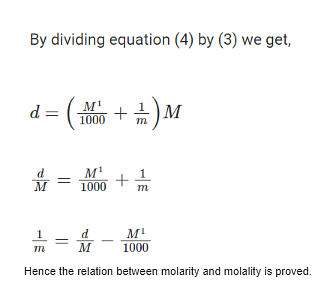
Differences Between Molarity and Molality
Both molarity and molality are the terms used to express the composition and concentration of a solution. Find below some of the basic differences between molarity and molality for your reference:
| Parameters | Molarity | Molality |
| Measure of | Concentration of the solution | Concentration of the solution |
| Defined as | The number of moles of the solute per litre of solution. | The number of moles of solute present in 1 kilogram of solvent. |
| Represented as | M | m |
| Equation/formula | Moles of solute/volume of solution (in litres) | Moles of solute/weight of solvent (in kg) |
| Represented as a ratio of moles to | Volume of solution in litres | Weight of solvent in kgs |
| Relation Between Molarity and Molality | M=1000d / [(1000 /m)+M1] | |
Basic Definitions
Both molarity and Molality are terms used to express the composition and concentration of a solution with respect to the solute and solvent. The basic definitions of various terms associated with molarity and molality are explained below:
Solute, Solvent, and Solution
- A solute is the component of a solution present in small quantities.
- A solvent is a component of a solution present in large quantities.
- The solution is a mixture in a liquid state comprising two components, i.e. a solute and a solvent. Both the solute and solvent mixture forms the solution.
Mole
A mole is defined as a standard scientific unit for measuring large quantities of very small entities such as atoms, molecules, or other specified particles. For example, 12 gms of Carbon-12 atoms represents one mole.
Molarity
Molarity, also known as molar concentration, is defined as the number of moles of the solute per litre of solution. It is denoted using the symbol M (capital m). The formula for molarity is expressed as
Molarity = moles of solute/litres of solution
Molality
Molality is defined as the number of moles of solute present in 1 kilogram of solvent. It is denoted using the symbol m (small m). The formula for molality can be given as
Molality = moles of solute/kilograms of solvent























POST YOUR COMMENT