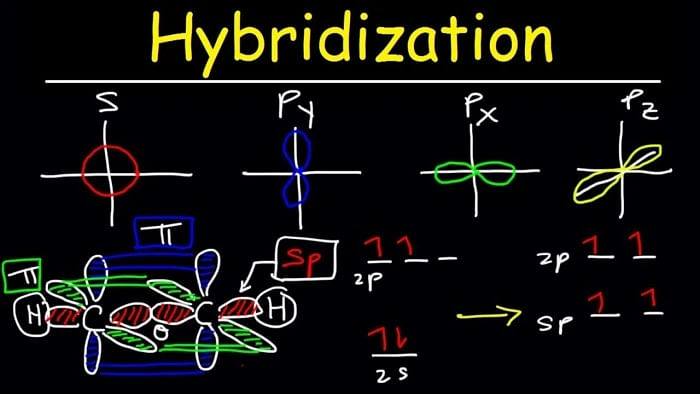
The concept of mixing atomic orbitals to form new hybrid orbitals is called Hybridization. Gain the knowledge and understanding of various hybridized orbitals along with important properties.
Table of Contents
In chemistry, hybridization is recognised as mixing two atomic orbitals to rise to a new type of hybridized orbitals. Further, there is a formation of hybrid orbitals having entirely different energies, shapes, etc., due to the intermixing.
Moreover, the atomic orbitals of similar energy levels are hybridization. Both the filled and half-filled orbitals can be involved in the hybridization process. However, they must possess equal energy.
What is Hybridization?
Hybridization is the process that involves the redistribution of energy of orbitals of each atom to produce orbitals of equivalent energy that happens when two or more atomic orbitals combine to form a hybrid orbital in a molecule.
Nonetheless, the hybrid orbital is used to explain atomic bond properties and molecular geometry.
Additionally, hybridization involves atomic orbitals of similar energies that are mixed. Generally, two 'p' orbitals or two 's' are mixed, or a single 'p' orbital with 's' orbital are mixed, or the mixing of 's' orbital with 'd' orbital happens. Hence, mixing different orbitals creates a new orbital known as hybrid orbitals.
Key Points of Hybridization
Let us see the key takeaways from hybridization:
- Hybridization gives rise to a new type of hybridized orbitals.
- Both half-filled and full-filled orbitals can be involved in the hybridization process.
- Further, the atomic orbital has the same energies that undergo hybridization.
- The number of hybrid orbitals formed equals the number of atomic orbitals mixing.
- Hybridization occurs exclusively during the bond formation and not in an isolated gaseous atom.
- The more prominent lobe of the hybrid orbital constantly has a positive sign. In contrast, the smaller lobe on the opposite side has a negative sign.
- The hybridization of the molecule can predict the molecule's shape.
Example:
Give the hybridized states of each of the carbon atoms in the given molecule.
H2C = CH – CN
HC ≡ C − C ≡ CH
H2C = C = C = CH2
Types of Hybridization
The hybridization can be classified into sp³, sp², sp, sp³d, sp³d², sp³d³, based on the types of orbitals engaged in mixing.

The points below discuss the different types of hybridization with examples.
1. sp Hybridization
When one 's' and one 'p' orbital in the same main shell of an atom mix to form two new equivalent orbitals, it is called sp hybridization.
- sp hybridization usually involves mixing one 's' orbital and one 'p' orbital of the same energy to produce a new hybrid orbital known as an sp hybridized orbital.
- The newly formed sp hybridized orbitals form linear molecules with an angle of 180°.
- sp hybridization is also called diagonal hybridization.
- Further, each sp hybridized orbital has 50% s and 50% p character, thus making it equal.
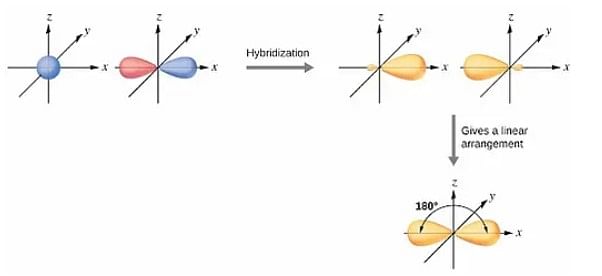
sp Hybridization Example:
- All compounds of beryllium like BeF2, BeH2, BeCl2
- All compounds of carbon-containing triple bonds like C2H2.
2. sp² Hybridization
When one 's' and two 'p' orbitals of the same shell of an atom mix to form ² equivalent orbitals, sp² hybridization occurs. Thus, the new orbitals formed are called sp² hybrid orbitals.
- sp² hybridization is also called diagonal hybridization; similarly, sp² hybridization is called trigonal hybridization.
- The process of sp² hybridization involves mixing one 's' orbital and two 'p' orbitals to form sp² hybridization.
- Moreover, sp² hybridization forms trigonal symmetry and is maintained at 1200.
- The three hybrid orbitals remain in one plane and make an angle of 120° with one another.
- Furthermore, the hybrid orbitals of 's' have 33.33% character and 66.66% character of 'p' orbitals. The molecules in which the central atom is linked to 3 atoms and is sp² hybridized have a triangular planar shape.
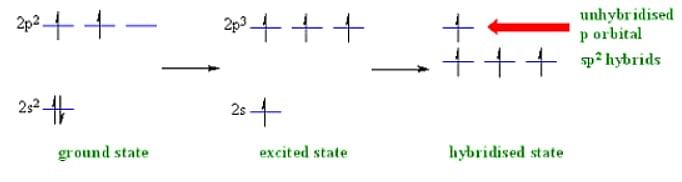
sp² Hybridization Example:
- All the compounds of Boron, i.e. BF3, BH3
- All the compounds of carbon-containing a carbon-carbon double bond, Ethylene (C2H4)
3. sp³ Hybridization
When one 's' and three 'p' orbitals of the same shell of an atom mix form 4 equivalent orbitals, sp³ hybridization of tetrahedral hybridization occurs.
- The new orbitals formed due to the mix of 4 equivalent orbitals are called sp³ hybrid orbitals.
- The process of sp³ hybridization involves mixing one 's' orbital and three 'p' orbitals to form sp³ hybridized .
- Additionally, the hybridized forms an angle of 109.5° and is directed towards the four corners of a regular tetrahedron.
- Nonetheless, the sp³ hybridization has 75% character of 'p' and 25% of 's'.
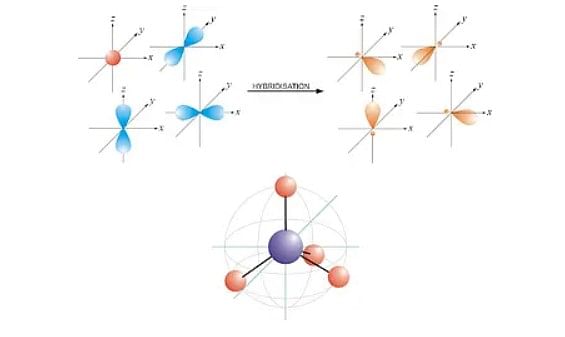
Example of sp³ Hybridization:
- Ethane (C2H6), methane.
4. sp³d Hybridization
When one 's', three 'p', and one 'd' orbitals of the same shell of an atom mix to form 5 equivalent orbitals of equal energy come together, it forms sp³d hybridization.
- When mixed, the 5 equivalent orbitals of equal energy are also called trigonal geometry.
- Next, three hybrid orbitals lie in the horizontal plane inclined at an angle of 120° to each other, known as the equatorial orbitals.
- However, the remaining two orbitals lie in the vertical plane at 90 degrees of the equatorial orbitals known as axial orbitals.
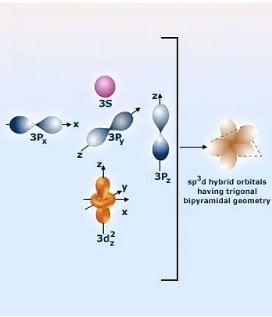
Example sp³d Hybridization:
- Hybridization in Phosphorus pentachloride (PCl5)
5. sp³d² Hybridization
The mixing of one 's', three 'p', and two 'd' orbitals of the same shell of an atom of equal energy comes together to form 6 identical sp³d² hybrid orbitals.
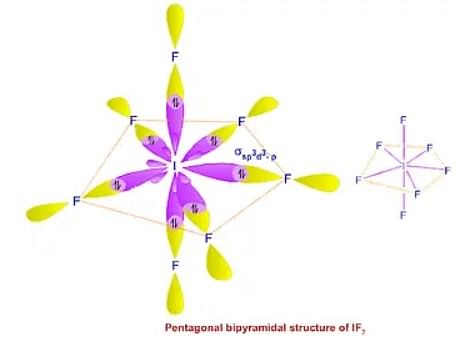
- sp³d² hybridization has 1s, 3p and 2d orbitals.
- Further, these 6 orbitals are directed towards the corners of an octahedron.
- They are inclined at an angle of 90 degrees to one another.
Hybridization: Important Properties
Now that there is a clear idea about hybridization, here are some of the key takeaways:
- The atoms of equal energy undergo hybridization .
- The mixing of different orbitals creates a new orbital known as hybrid orbitals.
- However, both the filled and half-filled orbitals can be involved in the hybridization process.
- Hybridization occurs only at some stage in the bond formation and not in an isolated gaseous atom.
- The hybridization process may predict the form of the molecule.
- The more prominent lobe of the hybrid orbital constantly has a high-quality sign, at the same time as the smaller lobe on the other facet has a bad sign.
Molecules and its Formation
Here are some of the molecular features:
| Methane (CH₄) | By involving C-sp³ with an H-1s, 4 equivalent C-H σ bonds can be formed. |
| Ethane (C₂H₆) | 6 C-H sigma σ bonds can be formed by the interaction of C-sp³ with an H-1s orbital, and 1 C-C sigma bond can be made by the interaction of C-sp³ with another C-sp³ orbital. |
| Sulfuric acid (H₂SO₄) | It has two hydrogen atoms, one sulfur atom, and four oxygen atoms. |
| Ammonia (NH3) and Water (H2O) | In the NH2 molecule, the nitrogen atom is sp³- hybridized, and one hybrid orbital possesses two electrons. To form an NH3 molecule, three 1s- orbitals of three hydrogen atoms overlap with three sp³ orbitals. The angle between H-N-H has to be 109.50, but as there is one occupied sp³- hybrid orbital, the angle decreases to 107.80. Therefore, the bond angle in the NH3 molecules is 107.80. |
FAQs on Hybridization
Q. What are the different types of hybridization?
A. The hybridization can be classified:
sp hybridization (beryllium chloride, acetylene)
sp² hybridization (boron trichloride, ethylene)
sp³ hybridisation (methane, ethane)
sp³d hybridization (phosphorus pentachloride)
sp³d² hybridization (sulphur hexafluoride)
sp³d³ hybridization (iodine heptafluoride)
Q. Explain the formation of sp, sp² and sp³ hybridization?
A. When one 's' and one 'p' orbital in the same main shell of an atom mix to form two new equivalent orbitals, it is called sp hybridization. Next, one 's' and two 'p' orbitals of the same shell of an atom mix to form ² equivalent orbitals, sp² hybridization occurs.
Furthermore, one 's' and three 'p' orbitals of the same shell of an atom mix to form 4 equivalent orbitals, sp³ hybridization.
Q. Why is BCl3 a planar molecule, whereas NCl3 is pyramidal?
A. It is because the BCl3 has no pair, but NCl3 has a lone pair of electrons.
Q. Explain the difference between molecular and hybrid orbitals.
A. The interactions between the atomic orbitals of two different atoms result in molecular orbitals. In contrast, when the atomic orbitals of the same atom interact, they form hybrid orbitals.
Q. What percentage of s and p characters are in sp, sp² and sp³ hybrid orbital?
A. The percentage of s and p character in sp, sp² and sp³ hybrid orbital is:
sp: s characteristic 50% and p characteristic 50%,
sp²: s characteristic 33.33% and p characteristic 66.66%,
sp³: s characteristic 25% and p characteristic 75%.






















POST YOUR COMMENT