RBSE Class 12 Chemistry Latest Syllabus 2025-26: Download Latest and Revised RBSE Class 12th Chemistry Syllabus PDF
Table of Contents
The RBSE 12th Chemistry syllabus 2026 includes chapters like Solutions, Electrochemistry, Chemical Kinetics, Amines, Biomolecules and more. The Chemistry syllabus contains 10 chapters, and the topics that have maximum weightage are Aldehydes, Ketones and Carboxylic Acids. The topic that has the minimum weightage is Biomolecules. The Chemistry syllabus contains vital information on the topics that need to be covered in the board exams, along with the marking scheme.
The RBSE 12th exams chemistry will be conducted for 100 marks, 70 of which are allotted to theory and the remaining to practicals. Students have to secure a minimum of 33 per cent to pass the Chemistry paper.
RBSE 12th Chemistry Syllabus 2026: Download
The RBSE 12th Chemistry syllabus 2026 will be released on the official website. The direct link to download the syllabus will be provided below for the student's reference.
| Particulars | PDF Link |
| RBSE 12th Chemistry Syllabus 2026 | To be updated |
RBSE 12th Chemistry Syllabus 2023-24: Download PDF
Students can access the RBSE 12th Chemistry Syllabus 2023-24 online. The syllabus is released in PDF format and incorporates essential information on the units to be covered and chapters that must be focused on priority. The PDF link to download the syllabus is given below.
| Particulars | PDF Link |
| RBSE 12th Chemistry Syllabus 2023-24 | Download Here |
Check for all subjects: RBSE 12th Syllabus 2023-24
RBSE 12th Chemistry Syllabus 2026: Course Details
The class 12 chemistry syllabus 2026 RBSE consists of 7 chapters and the marks weightage for every unit. Chemistry is one of the core subjects in science, and students must focus on completing the entire syllabus to ace the RBSE 12th exams. Important topics in the chapter are mentioned below.
| Topics | Name of the Unit |
| Solid State |
|
| Solutions |
|
| Electrochemistry |
|
| Chemical Kinetics |
|
| Surface Chemistry |
|
| p-block Elements |
|
| d and f-block Elements |
|
| Coordination Compounds |
|
| Halogen Derivatives |
|
| Functional groups with oxygen (Part-I) |
|
| Functional groups with oxygen (Part-II) |
|
| Organic compound containing functional groups with nitrogen |
|
| Stereochemistry |
|
How to Download the RBSE 12th Chemistry Syllabus 2026?
The RBSE 12th 2026 chemistry Syllabus is released online on the official website. The detailed steps to download the syllabus are given below.
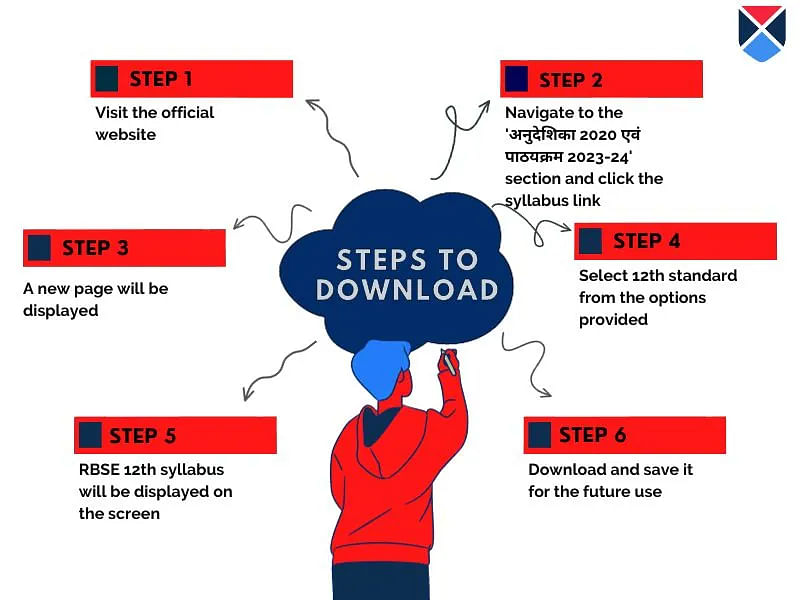
- Candidates must go to the official website at rajeduboard.rajasthan.gov.in.
- On the left-hand side panel, click on the 'अनुदेशिका 2020 एवं पाठयक्रम 2026'.
- Students will get redirected to a new page.
- Scroll down and select class 12th from the options provided.
- The RBSE 12th chemistry syllabus will be displayed on the screen.
- Candidates must download the PDF file and save it for future reference.
RBSE 12th Chemistry Syllabus 2026: Exam Pattern
Chemistry is one of the core science subjects, and the questions expected can get complex. Therefore, students should refer to the RBSE 12th 2026 Chemistry syllabus and prepare for the board exams.
As per the syllabus, students must focus on Aldehydes, Ketones, and Carboxylic Acids following the rest of the units. The marks allocated to every unit are given below.
| Units | Marks Allotment |
| Solutions | 6 |
| Electro Chemistry | 6 |
| Chemical Kinetics | 6 |
| d and f- block elements | 5 |
| Coordination Compounds | 5 |
| Haloalkanes and Haloarenes | 6 |
| Alcohols, Phenols and Ethers | 6 |
| Aldehydes, Ketones and Carboxylic Acids | 7 |
| Amines | 5 |
| Biomolecules | 4 |
Also read: RBSE 12th Exam Pattern 2024
RBSE 12th Chemistry Syllabus 2026: Important Points
The RBSE 12th Chemistry Syllabus 2026 aids the students appearing for the board exams by providing detailed chapters, unsolved questions, and mark allotment for vital units. Some essential points are listed below for the candidate’s reference.
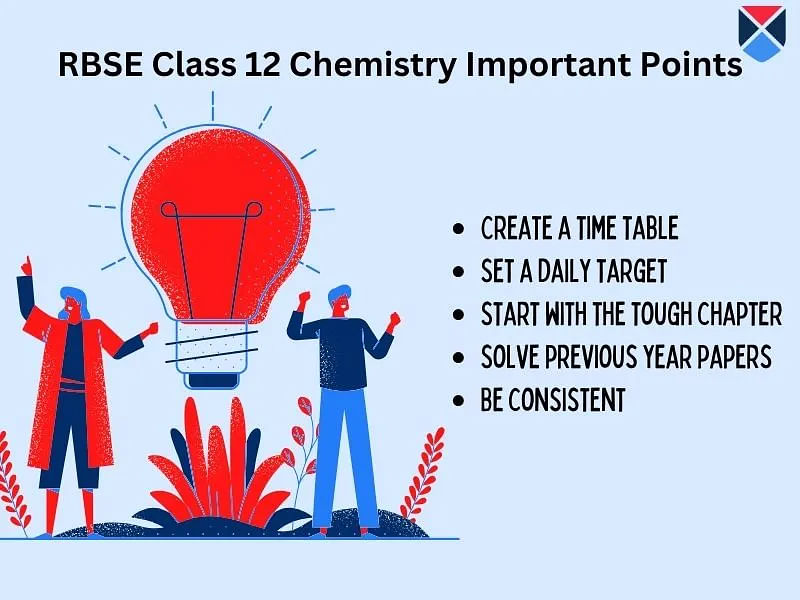
- Students must complete the whole class 12 chemistry syllabus 2026 RBSE syllabus so that they have time for revision.
- Create a study routine and focus on the chapters carrying maximum marks, followed by the rest of them.
- Practicing the RBSE 12th previous year question papers will help them understand the types of questions asked and the marking scheme.
- As most questions asked in the board exams are taken from the RBSE 12th 2026 chemistry syllabus, students must aim to finish the entire syllabus.
- It is advisable to ask the teacher’s guidance if there are any doubts.
Also read: RBSE 12th Books

![Maharshi Dayanand Saraswati University, [MDSU] Ajmer](https://media.getmyuni.com/azure/college-image/small/maharshi-dayanand-saraswati-university-mdsu-ajmer.webp)





![Ajmer Institute of Technology, [AIT] Ajmer](https://media.getmyuni.com/azure/college-image/small/ajmer-institute-of-technology-ait-ajmer.jpg)

![Aryabhatta College of Engineering and Research Centre, [ACERC] Ajmer](https://media.getmyuni.com/azure/college-image/small/aryabhatta-college-of-engineering-and-research-centre-acerc-ajmer.jpg)